What is Propane?
C3H8 is a three-carbon alkane with the chemical name Propane.
Propane is also called n-Propane, Dimethylmethane, or Propyl hydride. It is a gas molecular entity and acts as a food propellant. Propane was discovered in the year 1857 by Marcellin Berthelot who was a French chemist.
n-Propane is a colourless gas which has a faint petroleum-like odour. It is soluble in ethyl ether, chloroform, and benzene. It is usually obtained as a by-product of two other processes viz petroleum refining and natural gas processing. It is widely used as a fuel.
Table of Contents
- Properties of Propane
- Propane Structure
- Uses
- Chemical reactions of Propane
- Propyne and Propene can be distinguished by
- Health Hazards
- Frequently Asked Questions - FAQs
Properties of Propane - C3H8
C3H8 Propane Molecular weight of C3H8 44.097 g/mol Density of Propane 2.0098 kg/m3 Boiling point of Propane −42.25 to −42.04 °C Melting point of Propane −187.7 °CPropane Structure - C3H8
The exact mass and the monoisotopic mass of Dimethylmethane is 44.063 g/mol. The number of hydrogen bond acceptors and the number of hydrogen bond donors equals to zero.
C3H8 Uses (Propane)
- Propane is used in food additives.
- Used as a component in liquid petroleum gas.
- Used in the manufacturing of propylene and ethylene.
- Used as a fuel in cutting and welding operations.
- Used as a primary component for chemical synthesis.
- Used as a source of energy in motor vehicles, and water heaters.
- Used as improvised explosive devices.
- Used in lawn movers.
- Used in refrigeration.
- Used in the campaign.
- Used as an industrial fuel.
Chemical reactions of Propane
Like other alkanes, Propane also undergoes combustion reactions in a similar manner. Propane burns in the presence of an excess amount of oxygen to produce carbon dioxide and water.
C3H8 + 5O2 → 3CO2 + 4H2O + heat
When too much or too less oxygen is available for the combustion reaction, incomplete combustion takes place, forming soot (carbon) and/or carbon monoxide.
2C3H8 + 9O2 → 4CO2 + 2CO + 8H2O + heat
C3H8 + 2O2 → 3C + 4H2O + heat
The hydrogen content of Propyl hydride is extremely high and therefore burns hotter when compared to diesel fuel or home heating oil. The presence of C-C bonds causes it to burn with a flame.
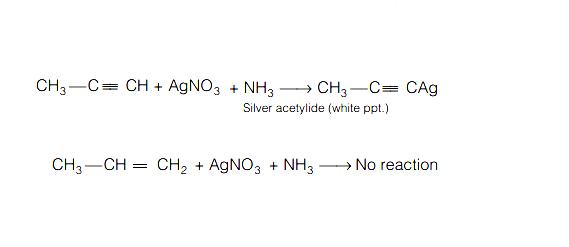
Propyne and Propene can be distinguished by
Propyne is terminal alkyne so it gives white precipitate with ammonical silver nitrate solution but alkene does not. Thus propene and propyne can be distinguished by ammonical silver nitrate solution. Terminal alkynes form silver salt with Tollen’s reagent while alkene does not react with Tollen’s reagent. Therefore, Tollen’s reagent can be used to distinguish a terminal alkyne like propyne from alkene as well as from internal alkynes.
Health Hazards
The vaporising liquid of propane may cause gangrene. It may cause dizziness if the concentration in air is greater than 10% and a higher dose causes asphyxiation. When heated it causes an explosion of containers. Its vapours are heavier when compared to air. This compound is extremely flammable. Learn more about the Structure, physical and chemical properties of C3H8 from the experts at BYJU’S.


